Difference Between Trigonal Planar and Trigonal Pyramidal
The atoms of a molecule are arranged in a number of ways to minimize the bond-bond repulsion, bond-lone pair repulsion and lone pair-lone pair repulsion. Molecules with the same number of atoms and lone pairs of electrons tend to exhibit similar geometrical shapes. The VESPR (Valence Shell Electron Pair Repulsion) theory is used to predict the geometrical shapes of molecules. As the name suggests, this theory predicts the shape of the molecule depending upon the number of electron pairs in the valence shell of the central atom. However, various spectroscopic methods and diffraction methods are also used to experimentally determine the molecular geometry of a molecule.
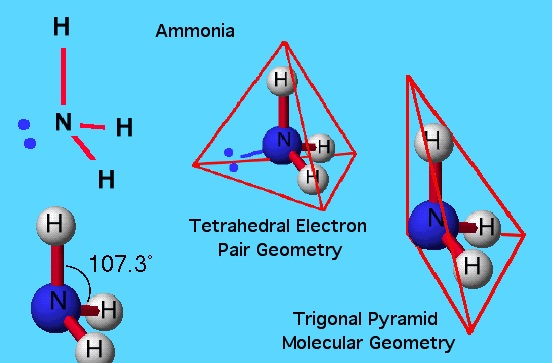
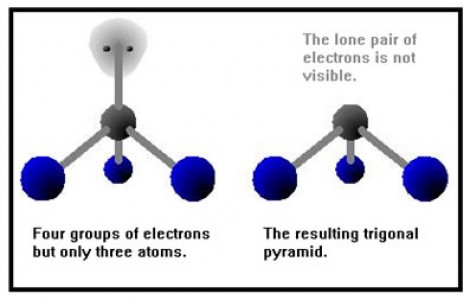
Trigonal planar and trigonal pyramidal are two of the several geometries that are used to describe the arrangement of atoms in a three dimensional plane. The central atom in a trigonal planar does not have a lone pair of electrons, whereas the central atom in a trigonal pyramidal has one lone pair or un-bonded pair of electrons. In the former, all atoms lie in one plane, while in case of the latter the atoms of the molecule do not lie in one plane.
Furthermore, the angle between bonded pairs of electrons in a trigonal planar is around 120 degrees whereas the bond angle in trigonal pyramidal is around 107 degrees. In a trigonal planar, only bond-bond repulsion determines the geometry of the molecule, while in the case of trigonal pyramidal, bond-bond as well as bond-lone pair repulsion is involved in the determination of the geometry of a molecule.
Instructions
-
1
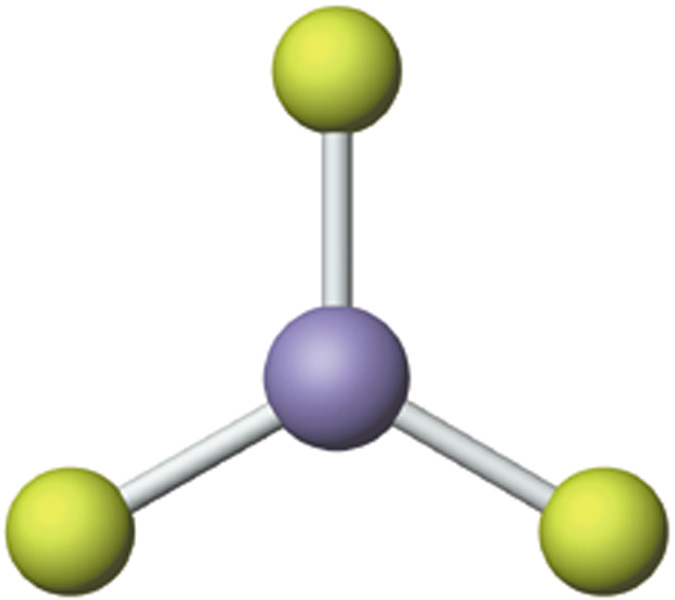
Trigonal Planar
Trigonal planar geometry is exhibited by the molecules in which four atoms have been covalently bonded together. One of the four atoms is the central atom whereas the other three are covalently bonded to it in such a way that they form corners of a triangle. The three atoms connected to the central atom are called peripheral atoms. In a trigonal planar, similar atoms are bonded to the central atom. Boron triflouride (BF3) is an ideal example of a molecule exhibiting trigonal planar structure.
Image courtesy: cwx.prenhall.com
-
2
Trigonal Pyramidal
Trigonal pyramidal geometry is also exhibited by molecules having four atoms; one central atom and three peripheral atoms. The central atom in a trigonal pyramidal is at the apex, whereas the other three atoms are at the base, with a bond angle of about 107 degrees. Ammonia NH3 is the best example of a trigonal pyramidal structure.
Image courtesy: elmhurst.edu