Daytrana; The ADHD Patch

As the most widely studied childhood disorder, ADHD treatments and programs remain at the forefront of pediatric and psychiatric professionals in addition to focus by school educators and parents. In small children, generally under the age of 12, parents often find the administration of ADHD prescribed oral medications are difficult as many children simply may not be able to, nor want to, swallow a pill. With the advance in pediatric ADHD treatment, the development of an ADHD patch, Daytrana, will allow for a more versatile approach to treatment in small children. Understanding the concept behind the patch, the side effects and contraindications will enable parents to make informed decisions regarding the care of a young ADHD child.
Daytrana, the ADHD patch manufactured by Noven Pharmaceuticals, is the size of a credit card. Offering nine continuous and sustained release hours of methylphenidate, the ADHD patch, Daytrana, is considered an ideal alternative to traditional oral pill forms of the methylphenidate, such as Ritalin and Concerta. Targeted primarily to children ages six to 12, the patch is expected to treat approximately 15 percent of the ADHD pediatric population. Placed at the hip location of the child, the disadvantage to the patch usage is in the ability of the child to remove the patch at any point of the day. However, with proper training, most children will find the therapeutic results are significant enough to result in non-removal of the patch.
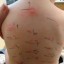
Previously rejected by the FDA in 2003 under a 12 hour sustained released patch, the new patch, under a nine hour sustained release, was approved by the FDA in 2006. With preliminary studies showing side effects including tics, insomnia and significant weight loss, the FDA has approved the modified version of the patch which shows to significantly reduce side effects as previously demonstrated. Of concern with the second trial is an indication of the development of a skin rash in the around in and around the Daytrana patch location on the hip. Although rare, the rash proved to be significant to cause concern. The panel reviewing the ADHD patch for the second review of approval has recommended the patch be utilized only as a second line of therapy in the event oral medications are not successfully administered or utilized in resolving the ADHD symptoms.
The use of Daytrana, the ADHD patch, is contraindicated in children suffering from anxiety, glaucoma and severe tension or agitation. In children presently treated for depression, the use of Daytrana may also be contraindicated and should be discussed with the pediatrician or psychiatrist prescribing the ADHD patch. Dosing is recommended in a 30 mg patch which should be removed immediately following a nine hour administration which provides for additional concern with regard to administration throughout the period of the school day.
For children suffering from ADHD symptoms, the use of methylphenidate provides significant results in terms of relieving ADHD symptoms while improving social, psychological and academic performance. For children unable to use oral medications, the use of Daytrana, the ADHD patch, may be considered as a second line of therapy. For more information, contact your child’s pediatrician regarding the use of Daytrana patches.