How to Make a Fruit Battery Science Fair Project
A reader may possibly think the title of this article has a typing mistake and how can a fruit be used to make a battery. This is because fruits, over the years, have been used to make salads, sauces and other such edible items. But making a battery with help of a fruit is certainly remarkable. For those who look upon this as something absolutely impossible, there is some authentic information about the fruits. According to that information some fruits, with lemon being the most common, can prove to be little power plants to make a fruit battery for a science project. For this reason, if you are a science student and looking for ideas regarding your middle school science project, then a fruit battery can certainly be one good idea.
After you finishing going through this guide, you will look at Lemons in a whole different way. Remember when life throws you a lemon, make a battery out of it. Continue reading below and have fun.
Instructions
-
1
Keep the following items readily available:
Things Required:
- Lemon
- Sand Paper
- Galvanized Nail
- Coin or piece of Nickel
- Voltmeter
Cleaning the Equipment
First of all you have to make sure that the equipments you are using are perfectly clean and there should be nothing on their surface which may hinder the flow of electricity. Rub the galvanized nail and the piece of nickel with sand paper so that they become clean and smooth. -
2
Rolling the Lemon
Now the lemon comes into play. Roll it under your hand on a table to make the juice loosen up but without damaging the peel. As the particles of the lemon move around due to rolling, it will result in creating energy.

-
3
Making Slits in to Lemon
Make two slits about one inch apart by slicing through the lemon. Be careful that these slits are neither too big and nor too small.

-
4
Inserting the Nail and Nickel
After the two slits that you have made in the previous step, insert galvanized nail into one and the piece of nickel to the other.

-
5
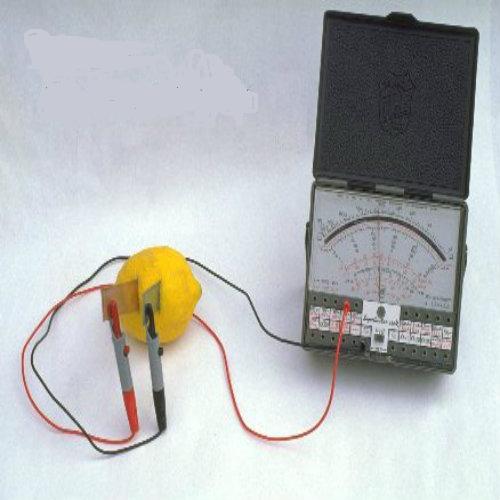
Connect a voltmeter
As you touch the inserted galvanized nail and nickel, you will feel the current flowing through. Connect the two electrodes of voltmeter; one with nail and the other one with the nickel. The deflection of needle will confirm the presence of current.