Human Battery Experiment Demonstration

You must have experienced an electric shock at least once in life. You may also have noticed that when you keep standing in the sun, your body gets charged up and if you shake hands with someone, either he or you will get an electric shock. It will not be fatal but will make the other person jitter. The demonstration of this principle can be used for a Science project if you are a middle school student. You simply need your index and middle finger to demonstrate this human battery, as substitutes to the salt bridge in a simple electrochemical cell.
Required Items:
– Two glass beakers or containers
– Copper Sulphate
– Zinc Sulphate
– Table Salt
– Distilled Water
– Zinc and Copper Strips
– U shaped plastic tube
– Cotton pieces
– Voltmeter
Instructions
-
1
The electrochemical Cell
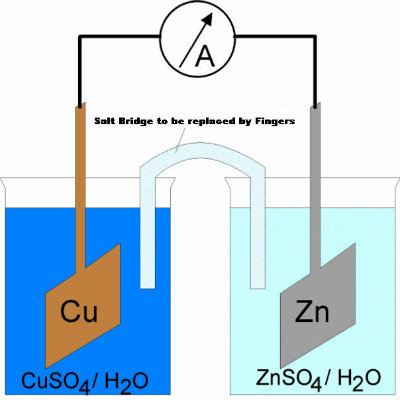
Using all the above items, first make an electrochemical cell. Simply take two beakers and fill one with copper sulphate solution and the other with zinc sulphate solution. -
2
The Salt Bridge
After filling the two beakers with different solutions, make salt bridge by dissolving some salt in water and putting it into a U shaped plastic tube. The tube, with ends inverted and covered in cotton pieces, will allow the flow of ions when each of its two ends are immersed in the beakers. Upon getting connected to the voltmeter, you will notice some deflection. -
3
Replacing the Salt Bridge with Fingers
Using your fingers, make a 'V' and dip one finger in the beaker of copper sulfate solution and other finger in the beaker of zinc sulfate solution. You will be surprised to see deflection in the voltmeter, since your body just worked as a battery! This 'battery' will approximately have similar voltage as an average cell does.