Difference Between Atom and Ion of the Same Element
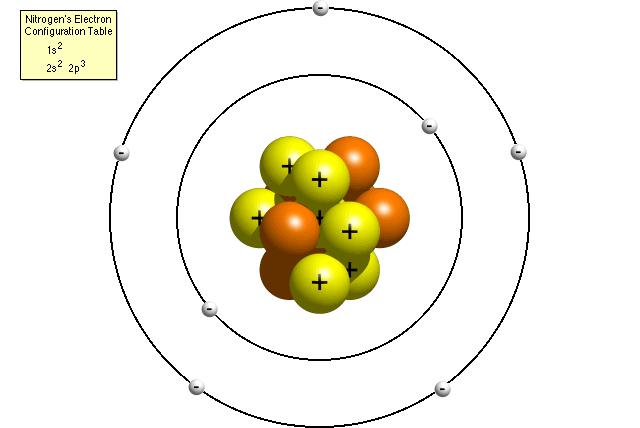
Chemistry is a very vast subject which starts with the knowledge of atoms and ions. Over years of scientific research, scientist came to the conclusion that atom is an indivisible unit of matter. Scientist also noticed the atom’s instability which is why it has to undergo a certain change before it could become chemically stable. This change results in the formation of ions. Atoms and ions are closely linked to each other. Differentiating between atoms and ions is a complex thing. Here are few basic differences between atoms and ions of the same element.
Instructions
-
1
An atom is electrically neutral while an ion is either negatively or positively charged. A positively charged ion is called a Cation whereas a negatively charged ion is called an Anion.
-
2
Atoms take part in molecular reactions while ions take part in a chemical reaction. Both are incapable of the contrary which means that there is no way an atom itself can take part in a chemical reaction. Similarly, an ion cannot combine with another ion by means of a molecular reaction.
-
3
The number of protons and electrons is equal for an atom of a particular element. On the other hand, there is a difference between the number of protons and electrons in an ion of the same element. An ion will have a positive charge if the number of protons is greater than the number of electrons and vice versa.
-
4
An atom becomes an ion by loosing or accepting one or more electrons in its outermost shell. Atoms of all elements except the noble gases have to undergo this change because an atom itself does not meet the duplet or octet rule of chemistry.
-
5
Combination of atoms results in a molecular bond whereas combination of ions results in an electro-covalent bond. Furthermore, molecular bonds are much weaker than electro-covalent bonds.
-
6
All atoms except noble gas elements cannot exist in independent state. They have to gain or lose electrons in order to gain stability. Ions on the other and are conventionally stable and can exist in combination with another ion.
-
7
All atoms except halogen gas atoms do not have 2 or 8 electrons in their outermost shells. Ions on the other hand meet this condition my accepting or donating electrons from or to another atom of an atom of another element.