Difference Between Ionic and Molecular Compound
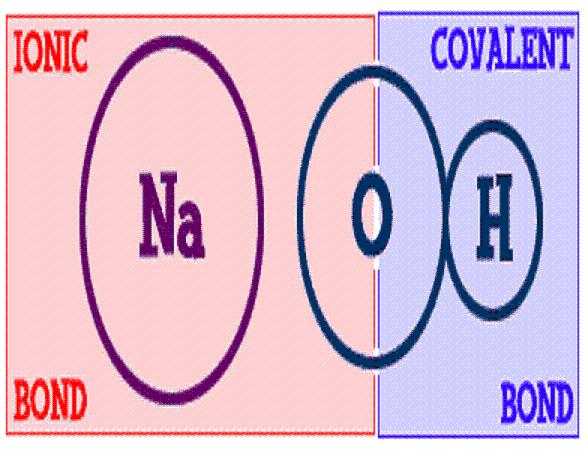
Compounds can be of two types, i.e. ionic and molecular. Even though both the compounds involve atoms joined together, molecular compounds are made of two or more neutral atoms joined together by sharing electrons, whereas ionic compounds are formed by the joining of electrically-charged atoms. Covalent bonds are involved in molecular compounds, while ionic compounds are a result of ionic bonding. Ionic compounds are formed between non-metals and metals, whereas molecular compounds are formed between two non-metals. Ionic compounds are much better conductors of electricity than molecular compounds. Molecular compounds can be in solid, liquid or gas state, whereas ionic compounds are in either solid or crystalline form. Molecular compounds are far more common than ionic compounds.
Instructions
-
1
Ionic Compounds
Ionic compounds are formed when two or more electrically charged atoms join together. The atoms become electrically charged by losing or gaining an electron, becoming positively or negatively charged as a result of the change in the ratio of protons in the nucleus and number of electrons in the shells around it. The bonding involved in the joining of electrically charged atoms is called ionic bonding. Ionic compounds are formed between metals and non-metals. Metals are more than willing to let go of the electrons in their outer-most shell, becoming positively charged as a result, while non-metals are willing to receive an electron in their outmost shell. Having free and extremely mobile electrons make ionic compounds good conductors. The strong attraction between the electrically charged atoms end up giving ionic compounds a solid or crystalline form. Salt is one of the many commonly seen examples of an ionic compound, which is formed by the combination of sodium and chlorine.
Image Courtesy: apchemcyhs.wikispaces.com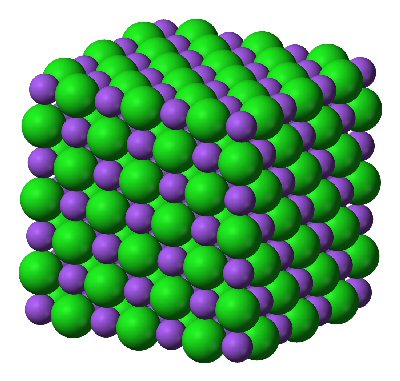
-
2
Molecular Compounds
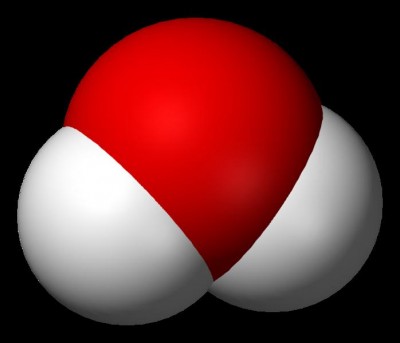
Molecular compounds are formed when two or more neutral atoms, i.e. having similar number of protons and electrons, join together by sharing the electron or electrons in their outer-most shell. The form of bonding between these atoms is known as covalent bonding. Molecular compounds are formed between two or more non-metals. Molecular compounds do not have free electrons, which consequently make them really bad conductors. The strength of bonds between atoms in molecular compound can vary, which is why they can commonly be found in solid, liquid or gas state. Water is an example of molecular compound.
Image Courtesy: chemistry.about.com