Difference Between a Pure Substance and a Mixture
Generally matter is classified in the form of states – solid, liquid and gas.
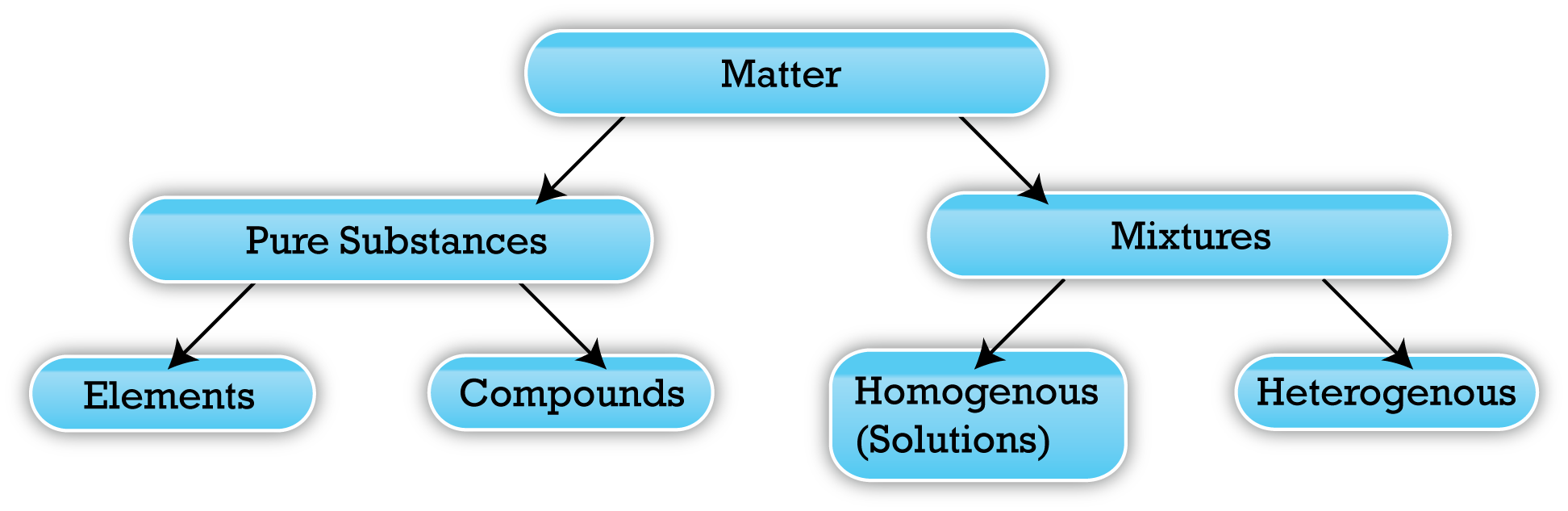
It can however also be classified in the form of composition – pure substance and mixtures.
There are a number of differences between pure substance and mixture, the most evident being that pure substances are made up of a single particle of matter, whereas mixture is a combination of two or more pure substances or in other words, particles of matter.
Instructions
-
1
Pure Substance
A pure substance has the following main characteristics;
- It cannot be separated into two or more than two substances through any physical and chemical means.
- These substances have the same chemical composition and properties throughout. There is no heterogeneity.
There are two types of pure substances, elements and compounds.
An element is a pure substance which is composed of a single type of atom, an entity which is the smaller unit of matter which retains its chemical properties and composition. Atom is in fact the building block of any elements, which in turn are the building blocks of matter. An example of element is gold, silver, iron and so on.
A compound is a mixture of two or more elements in a specific ratio. For example, water contains hydrogen and oxygen in a ratio of 2:1. Each compound has its unique properties which are constant throughout. These types of pure substances are very hard to separate, but it can be done through extensive chemical reactions and separation processes.
Image courtesy: birdieshealthchatter.com

-
2
Mixtures
A mixture has the following characteristics;
- They can be separated through physical and chemical means.
- They do not have a uniform composition throughout, and it can be varied by altering the amount of pure substances involved in the composition.
- The chemical and physical properties of compounds can be altered.
Just like pure substances, mixtures are also of two types, homogeneous and heterogeneous.
Also called a solution, a homogeneous mixture has nearly the same composition throughout, for example, a solution of salt and water, or a solution of sugar and water.
A heterogeneous mixture is one which has different consistency and composition at different positions. For example consider a mixture of salt, flour and sugar in a jar. No matter how many samples you take, there is very little probability that any two samples will have the same composition. Such mixture is called heterogeneous.
Image courtesy: toxicology.org







