Difference Between Compound Elements and Atoms
Atom and element are the two basic terminologies used in chemistry. They both are the basic building blocks of matter and this is the reason beginners often mistakenly confuse them and think they both are the same. While an atom is the smallest particle of matter, an element is a pure chemical substance consisting of a number of similar atoms.
An element can be seen with the naked eye, while an atom is so small that they cannot be seen even through a microscope. More precisely, elements are specific substances, whereas an atom is the smallest unit that can exhibit the properties of a particular element. For example, iron is an element represented by the symbol Fe, a block of element exhibits the same properties as do the iron flakes. If you further break down it into atoms, every single atom will exhibit all the properties of iron, from electric conductivity to magnetism. However, an atom of aluminium and an atom of iron will exhibit different properties.
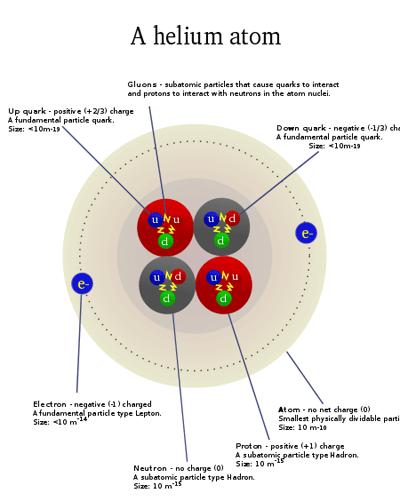
An atom can be further divided into three parts, a central nucleus containing protons and neutrons and electrons revolving around the nucleus. Protons bear positive charges while electrons bear negative charges and neutrons are neutral. An Element on the other hand, is the simplest form of matter, that cannot be split into simpler substances.
Instructions
-
1
Elements
An element is a pure chemical substance comprising of one type of atoms. Elements are distinguished by their atomic number, derived from the number of protons present in the nucleus of an atom of a particular element. There are 118 known elements, 98 of them occur naturally on the earth while the remaining have been produced artificially during nuclear reactions. The 98 naturally occurring elements are further divided between metals, non-metals and metalloids, on the basis of their chemical and physical properties. When two different elements combine chemically, they form a chemical compound. Helium and hydrogen, present at the top of the periodic table, are the lightest chemical elements.
Image courtesy: physicsmiracles.blogspot.com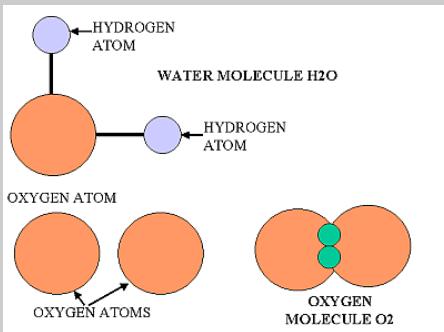
-
2
Atoms
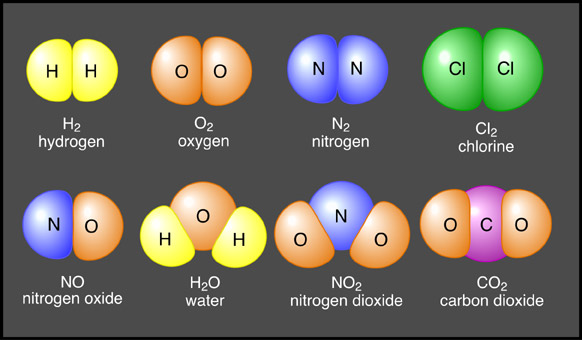
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. Atoms of similar or different elements come together to make molecules and compounds. For example two hydrogen molecules combine together to form a hydrogen molecule, while hydrogen and oxygen atoms combine chemically to make a new compound - water, that exhibits properties totally different from those of hydrogen and oxygen.
Image courtesy: differencebetween.net