Difference Between Viscosity and Density
Properties of matter vary in its different form and a basic knowledge of these properties can allow you to understand the behaviour of matter under certain circumstances. Solids, liquids and gases have entirely different physical properties but there are certain attributes which may differ in the same state of matter. All liquids might not have similar sort of physical properties and these differences might be helpful in choosing the right liquid for particular tasks. Density and viscosity of liquids are two properties of matter which are quite similar to one another but have many differences as well. These two physical properties are often mixed up with one another and you should be able to distinguish between them.
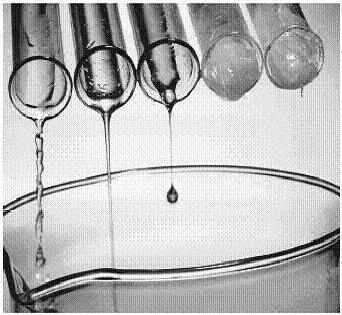
Viscosity is actually the resistance to gradual deformation by shear stress or tensile stress. As for the liquids, it can be defined as the resistance to flow. Thicker the liquid, more viscosity it possess. An excellent example is of honey and water. Honey flows very slowly and is highly viscous, whilst water flows rapidly and has a low viscosity.
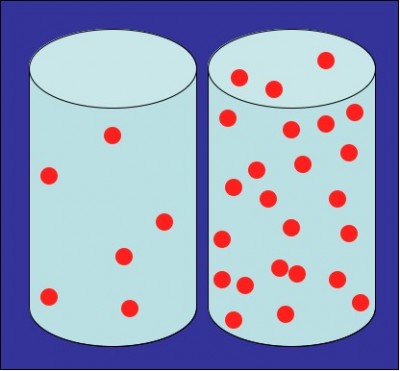
Density of a material is the mass per unit volume or weight per unit volume. It relates to the number of atoms or molecules of a substance present in a particular volume. Highly viscous liquids have very high density while lesser viscous liquids possess low mass per unit volume.
Instructions
-
1
Viscosity
Albeit it is a fact that denser liquids are more viscous, these two terminologies are not related in any meaningful way. It has also been seen that equally dense liquids have different levels of viscosity. Viscosity depends upon the friction between the layers of a substance. If the friction is very high, the liquid will tend to flow slowly and has a higher viscosity. The vital difference between density and viscosity is the effects of temperature. A substance’s viscosity can dramatically change when heated, whereas its density remains the same. Viscosity is mostly referred to define the movements of liquids or solids in molten form.
Image Courtesy: zeitnews.org
-
2
Density
Higher density of a liquid certainly increases its viscosity but this resistance to flow is susceptible to change at different temperatures. Density of a substance doesn’t change with rising temperature but viscosity can change significantly. Density is not referred to liquids only but to solids and gases as well. This attribute of a substance remains the same, regardless of the physical state.
Image Courtesy: spacegrant.montana.edu