Difference Between Strong and Weak Acids and Bases
Chemistry is about acids and bases reactions all the time. It might get boring and really confusing at times to distinguish between the strengths and weaknesses of these two chemical compounds. Not all the acids or basses tend to react vigorously, as their strength and reactivity varies. As a student of chemistry, you need to know the standards of determining the strengths of both acids and bases. Acids are chemical compounds which liberate H+ ions when dissolved in aqueous solutions, whilst bases are the compounds which release OH- ions when dissolved in water. It is the concentration of H+ and OH- ions which determines the strength of acids and bases.
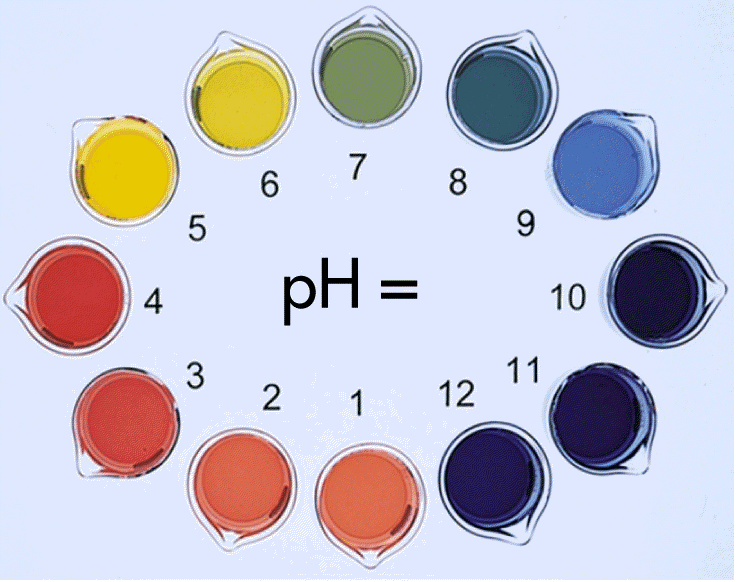
Acids which fully ionise in aqueous solutions, releasing maximum number of hydrogen ions, H+, (also referred as protons) are said to be strong acids. Those acids which only partially ionise in water are considered to be weak acids. Strong acids have a pH value far below 7 but weaker acids lie closer to 7 on the pH scale. The acidity merely determines the speed of reaction an acid has with other chemicals but the chemical properties of strong and weak acids are pretty much the same.
Bases which fully ionise and liberate hydroxide ions (OH-) when dissolved in water are called strong bases. Those bases which do not fully ionise in the aqueous solution are considered to be weak bases. The pH value to strong bases is closer to 14 and the weaker ones lie closer to 7 on the pH scale.
Instructions
-
1
Strong and Weak Acids
Acidic strength all depends upon how much an acidic compound ionises in water and how many protons it releases. Ionic compounds, like hydrogen chloride (HCl), completely break down into its constituent ions, H+ and Cl-, when dissolved in water. The presence of high concentration of H+ ions makes the aqueous solution very strong acid. On the other hand, when covalent compounds, like hydrogen carbonate (H2CO3), are dissolved in water, merely few of its molecules break up to form ions, H+ and (CO3)2-.
Image Courtesy: human-academy.com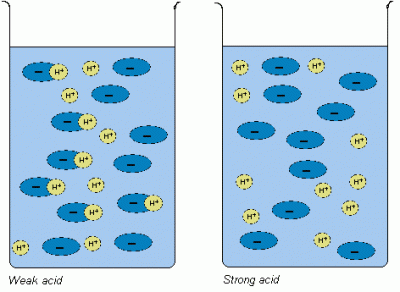
-
2
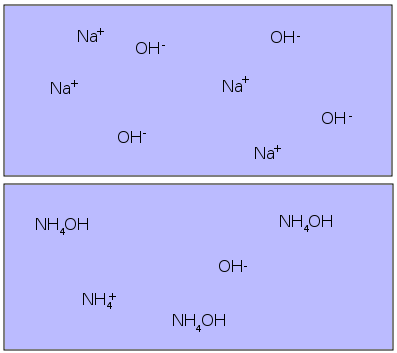
Strong and Weak Bases
The basic strength goes the same way as acidic strength i.e. the more OH- ions in the aqueous solution, stronger the base and vice versa. Ionic compounds, like sodium hydroxide (NaCl) readily ionise in water to release plenty of OH- ions, while covalent bases merely produce few hydroxide ions to form weak basic solutions. Strong acids and bases react extremely fast to form salts. However, a strong acid and a weaker base are considered to be a conjugate of one another and the salt formed by reacting these two compounds is a weak base as well.
Image Courtesy: water.me.vccs.edu