Difference Between Sulphuric and Hydrochloric Acid

An acid is a chemical compound that reacts with a base to produce salt and water. Acid is defined as a chemical substance that donates a proton (H+ ion) in an aqueous solution. All acids have a sour taste; vinegar and lemon juice are the most common acids used in daily life. Acids can be divided into two groups on the basis of their ability to produce protons when dissolved in water; strong acids and weak acids. Strong acids are the one that completely ionize in water to produce protons. Weak acids, on the other hand, partially dissociate in water and produce fewer amounts of H+ ions.
Hydrochloric acid and Sulphuric acid are the most common acids used in laboratories as well as in chemical industries. They both are strong acids, but Hydrochloric acid contains only one hydrogen atom while Sulphuric acid has two hydrogen atoms. Hydrochloric acid contains one chlorine atom, while Sulphuric acid contains one sulphur and four oxygen atoms.
Hydrochloric acid is a monoprotic acid, while Sulphuric acid is a diprotic acid, i.e. the former gives just one proton when dissociated in water whereas the latter produces two protons in aqueous solution. Molar mass of Sulphuric acid is 98.079 g/mol whereas the molar mass of Hydrochloric is 36.5 g/mol. The number of replaceable H+ ion in an acid determines the basicity of that acid. Hydrochloric acid is monobasic while Sulphuric acid is dibasic.
Instructions
-
1
Hydrochloric acid
Hydrochloric acid, represented by chemical formula HCl, is a clear, colourless, non-flammable mineral acid, which is highly corrosive. HCl is stable at room temperature but reacts readily with bases and metals. It ionizes in an aqueous solution to donate one proton (H+) or hydronium ion (H3O+). The dissociation reaction of hydrochloric acid in the aqueous medium is shown by following equation:
HCl +H2O → H3O+ + Cl-
- Image courtesy: chemistry.about.com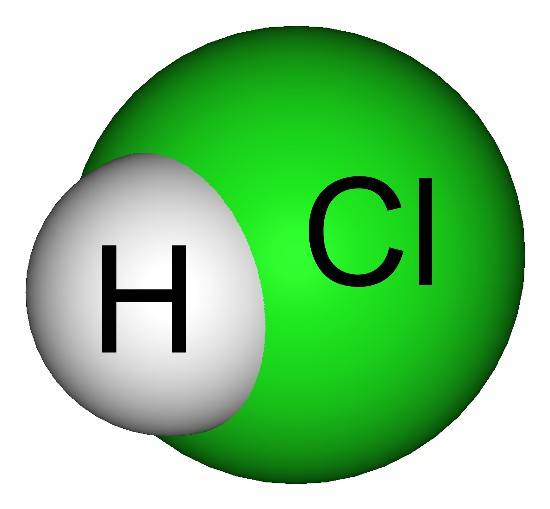
-
2
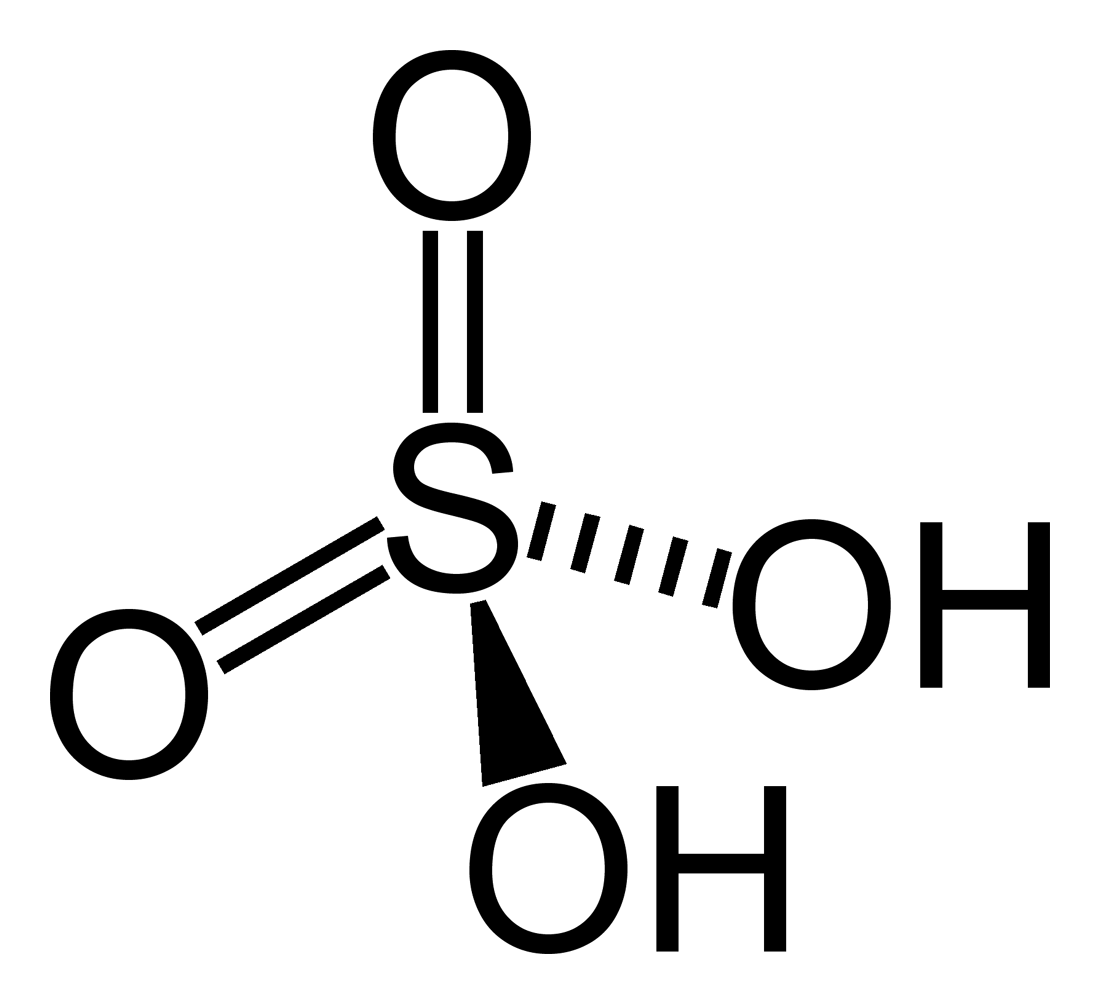
Sulphuric acid
Sulphuric acid, represented by chemical formula H2SO4 is a strong acid in which hydrogen, sulphur and oxygen atoms are arranged tetrahedrally. It is a clear, colourless, odourless, viscous liquid with density 1.84 g/cm3 and viscosity 26.7 cP (20 °C). Like all other strong acids, it is highly corrosive. Besides being a strong proton donor, it also acts as a strong dehydrating agent and this is the reason it is widely used in various condensation reactions. The ionization of Sulphuric acid in an aqueous solution is as follows:
H2SO4 → HSO41- + H+
HSO4 - → SO42- + H+
- Image courtesy: worldofchemicals.com