Difference Between Sodium Bisulfite and Sodium Metabisulfite
Sodium bisulfite and sodium metabisulfite are both salts of sodium, commonly used as food additives, as preservatives in cosmetics and pharmaceuticals, and at times as disinfectants as well.
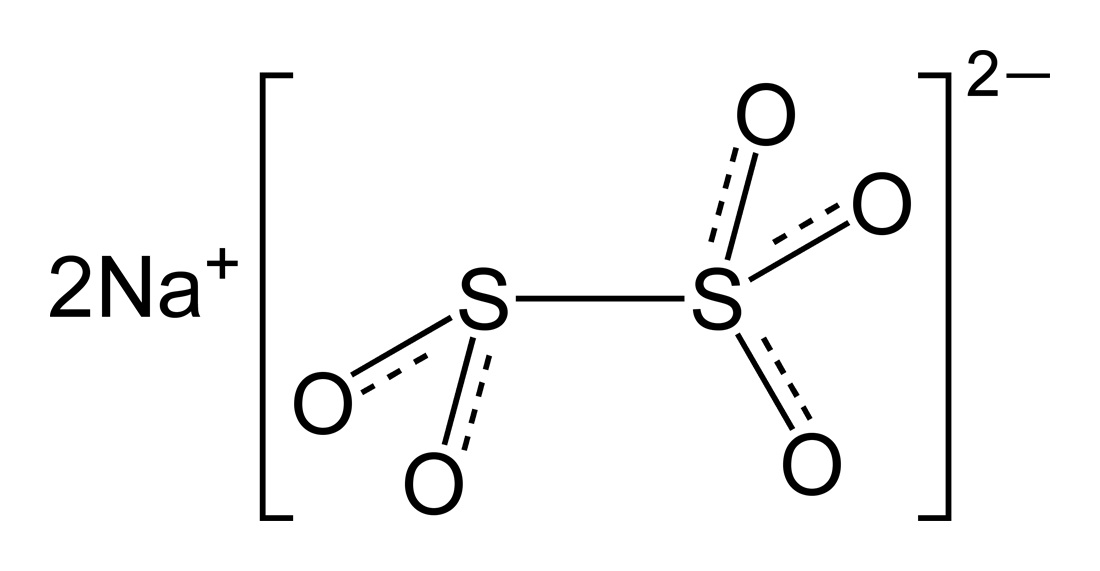
Sodium bisulfite is a sodium salt of sulphurous acid, in which three oxygen atoms are attached to one sulphur atom and the bisulphite ion is monovalent – that is, it has “minus one” (-1) valency. Sodium metabisulfite is also a sodium salt of sulphurous acid, but it has five oxygen atoms attached to two sulphur atoms, and the bisulphite ion is also divalent – that is, it has “minus two” (-2) valency.
Sodium bisulfite has a foodstuff additive number E222, while sodium metabisulfite has a food additive number E223. When dissolved in water, sodium metabisulfite changes into sodium bisulfite. Furthermore, in a chemical reaction, sodium metabisulfite gives more sulphite ions than sodium bisulfite.
Instructions
-
1
Sodium bisulfite
Sodium bisulfite is an inorganic chemical compound with the chemical formula NaHSO3. It is a salt of sulphurous acid which is prepared by bubbling sulphur dioxide gas through an aqueous solution of sodium carbonate. Two oxygen atoms and a hydroxyl anoin are directly attached to a sulphur atom, whereas the sodium ion is attached to an oxygen atom. The oxidation of sulphur in sodium bisulfite is +4; it has a molar mass of 104 grams per mol and a melting point of 150 degrees Celsius.
Sodium bisulfite naturally occurs as a white solid, which is soluble in water, and when dissolved in water, it forms a corrosive liquid. Sodium bisulfite is slightly acidic in nature, since it has the ability to donate a proton. When allowed to react with chlorine bleach (aqueous solution of sodium hypochlorite), sodium bisulfite forms hazardous fumes. This chemical has several uses in organic chemistry; for example, it reacts with certain cyclic ketones to form sulfonic acids, and in contact with aldehyde groups, it forms bisulfite adduct. When allowed to react with oxygen, sodium bisulfite oxides to form sodium bisulphate.
Image courtesy: commons.wikimedia.org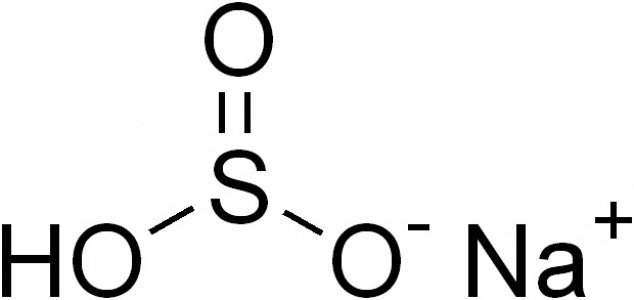
-
2
Sodium metabisulfite
Sodium metabisulfite, also known as sodium pyrosulfite, is a salt of sodium with the chemical formula Na2S2O5. The molar mass of sodium metabisulfite is 190 grams per mol, with three oxygen atoms attached to one sulphur atom and two oxygen atoms attached to another sulphur atom, and the whole radical is attached to sodium ion. Sodium metabisulfite naturally occurs as a white crystalline powder and is completely soluble in water. It has a melting point equal to 150 degrees Celsius. When dissolved in water, sodium metabisulfite produces sulphur dioxide gas.
Image courtesy: commons.wikimedia.org