Difference Between Sublimation and Deposition
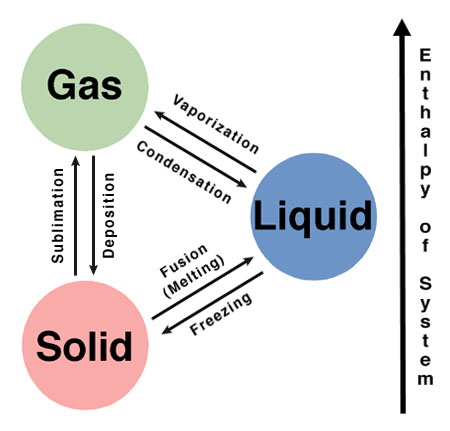
Sublimation and deposition are two phase transitions which actually change the state of a substance, for example solid changing to liquid or gas, or a liquid or gas changing to a solid state. This change in phase is mostly caused by the external factors like change in temperature or pressure. For example, a liquid converts into a gas when the temperature is raised to boiling point, or it may attain a solid phase when the temperature is reduced to freezing. Likewise, you can liquefy a gas by increasing the pressure.
Sublimation and deposition are not just phase transitions but they are a bit different than the usual changes that take place around us in our daily life. They are more extreme changes that the matter can undergo. Both processes occur naturally in the environment and play a key role in regulating the temperature of the world.
Both sublimation and deposition are converse to each other. Sublimation is a process by which a solid changes directly into a gas, without going through the liquid stage in the process. In deposition a substance changes directly from the gaseous state into a solid state bypassing the liquid state in between.
Sublimation is an endothermic process, which means heat is required by the system, while deposition is an exothermic reaction as heat is evolved by the system.
The sublimation process requires an environment with high pressure as well as high temperature (much higher than the melting point of the substance undergoing the sublimation process). The deposition, on the other hand, requires low temperature, low pressure environment.
The former normally occurs in the areas that experience extremely dry weather and abnormal temperature shifts, whereas, the latter takes place usually in the clouds where water vapours undergo a deposition process and convert into ice particles, that falls as snow.
Instructions
-
1
Sublimation
Sublimation is the phase transition of a substance directly from a solid state to a gas state, without undergoing an intermediate liquid state. Any substance can undergo sublimation, given the conditions (temperature and external pressure) are suitable. It is an endothermic process in which the substance takes heat energy from the environment all through the process.
- Image courtesy: commons.wikimedia.org
-
2
Deposition:
Deposition, also known as desublimation, is the is opposite of sublimation. It is a thermodynamic process in which gas transforms into a solid, without undergoing the liquefaction stage. For deposition to occur, heat energy must be removed from the substance undergoing the process. During deposition, the gas releases a substantial amount of energy into the environment.
- Image courtesy: mamontoff.org







