Difference Between Sublimation and Heat Transfer
Sublimation and heat transfer are both processes in which a transfer of medium is taking place. There are many differences between the two processes, the biggest one being that sublimation can be considered as the subset of the heat transfer process. These two parameters have been defined and explained in detail in this article, and their differences are also highlighted.
Sublimation is basically a change of state of matter, from solid to gas, where as heat transfer deals with the change of state in temperature (energy). The change in temperatures involves the transfer of heat from one body to another. This heat can be converted to another form of energy, and then transferred, but that is not possible in case of sublimation.
The basic understanding is very important in understanding and applying the principles of thermodynamics.
Instructions
-
1
Heat transfer
This is one of the major subjects of thermodynamics and its application involves literally every modern invention. This process is the basic part of nature and unknowingly takes places in our bodies at all times.
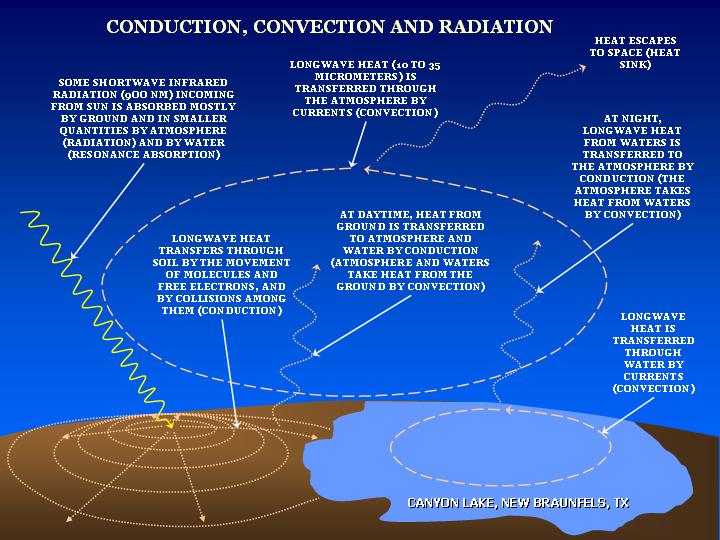
Heat transfer involves the transfer of heat from one body to another.
The question here is – what is heat?
Heat is the energy which is produced due to the interaction of molecules within a system. The atoms in these molecules have kinetic energies, due to which they collide with each other and the wall of the medium and release energy in the form of photons. Greater the photons in a system, greater will be its randomness and higher will be its temperature.
When this system makes a contact with another system which has lesser temperature, to create a thermal equilibrium the photons move towards it, resulting in heat transfer. This is the basic rule of thumb that the heat flows from the object with high temperature to an object with low temperature. In other words, there will be no heat transfer without a temperature gradient.
The amount of heat within a system is measured in Joules (J) under SI system, while the rate of change of heat is measured in Watts (W).
Image courtesy: aos.wisc.edu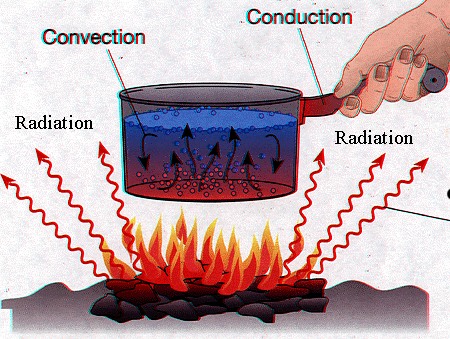
-
2
Sublimation
When a solid is heated to its melting point, the normal course of action is that it will melt into a liquid and when further heated, it will change its state to gas. In certain special materials like pure naphthalene and solid carbon dioxide, the solid directly changes into gas directly and this phenomenon is called sublimation.
Image courtesy: all-water.org