Difference Between Thermodynamics and Kinetics
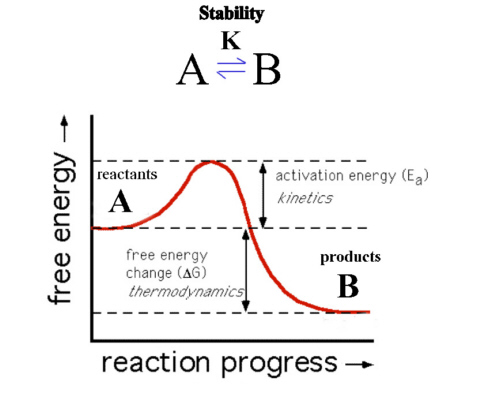
Kinetics and thermodynamics are both extremely important components of physical and chemical sciences and their applications are evident in almost anything which moves. There are quite a few pronounced differences between the two scientific principles.
When a body goes from one state into another, thermodynamics defines the net energy balance of the process and the equilibrium situation – static, dynamic, quasi static etc – whereas kinetics defines the speed with which the body changed its state or its rate of change.
Similarly in chemical sciences, kinetics indicates the speed of the reaction whereas thermodynamics is more concerned about its parameters, like how far has the reaction gone, was it exothermic or endothermic etc.
Instructions
-
1
Thermodynamics
Thermodynamics is derived from the thermo – meaning heat – and dynamics – meaning motion or change in motion. Hence thermodynamics can be defined as the branch of science which deals with the changes in temperature.
Talking more specifically, it is the branch of science which deals with energy, its transfer, change in its forms, and dissipation. Considering that there are so many types of energy, the spectrum of this branch is huge and covers everything which transmits heat or does some work.
Work is done when energy can be used to produce motion, and when there is a change of state due to temperature difference, we can say that there has been a flow of heat. Thermodynamics gives no indication about the speed at which the work has been done or the heat transfer has taken place.
Its applications are also wide spread in chemical sciences. In chemistry, thermodynamics can define the feasibility of the reaction, but cannot indicate the rate at which it will take place.
Image courtesy: fisitech.files.wordpress.com
-
2
Kinetics
Kinetics is basically concerned with the rate of change of states which any medium experiences. In a chemical reaction, it determines whether the reaction will take place at an appreciable and workable pace or not.
Kinetics depends on the energy of molecules within a medium. These molecules have a motion of their own and transfer energy by colliding with each other and the walls of the barrier. The amount of energy required to break this barrier is called the activation energy and when it breaks a chemical reaction can initiate. This energy of the molecules can be increased by increasing the temperature or by using a catalyst, which lowers the activation energy.
Determining which route is better and how the process should take place, comes under the scope of kinetics.
Image courtesy: massageking.com








