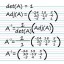Difference Between Titration and Back Titration
Titration is a chemical process which is widely used in analytical chemistry to determine oxidants, metal ions, reductants, acids and bases. It is simply a chemical reaction in which an analyte reacts with a standard reagent, that is called the titrant, to determine the concentration. On the other hand, back titration is a process in which an additional amount of standard titrant is added in order to determine the quantity of the analyte.
In titration, only one direct chemical reaction takes place, whereas in back titration, two chemical reactions occur at the same time.
Instructions
-
1
Titration
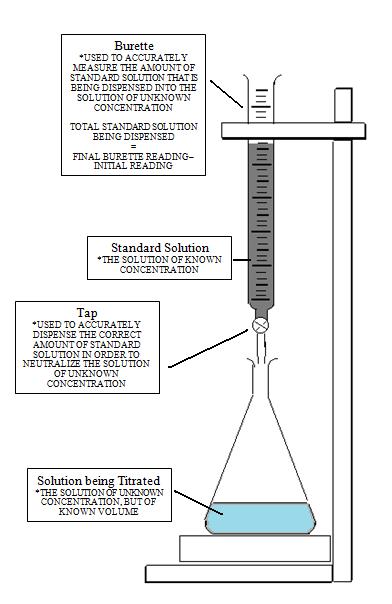
It is a quantitative chemical analysis technique which is used to assess the unknown concentration of a recognized analyte. It is also known as titrimetry and volumetric analysis. The measurements of volume play a crucial role in the process. The standard solution which is prepared during the reaction is known as a reagent. In order to determine the concentration, the titrant reacts with the solution of titrand or analyte.
Sometimes a purified and stable solution, known as primary standard, is used in titrimetric methods as a reference. To determine the quantity of the analyte, the volume of the titrant (required to complete the reaction) is identified. The analyte is poured into the titration flask with the help of a pipette. The whole chemical reaction takes place in the flask.
When the reaction is completed, it is detected by the indicator. Furthermore, the point at which the reaction is finished is known as the end-point. To detect and record the responses from the chemical reaction, different instruments are used which mainly include conductivity meters, turbidimeters and temperature monitors.
Image courtesy: sparknotes.com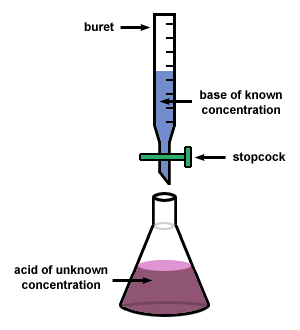
-
2
Back Titration
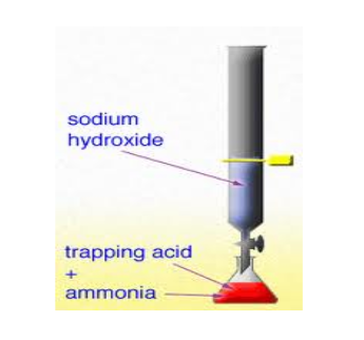
It is a chemical process in which a surplus quantity of the standard titrant is dropped into the analyte. A chemical reaction occurs between some quantity of the standard titrant and the analyte while the excessive quantity is measured by back-titration. For instance, to determine the quantity of phosphate, silver nitrate is added into the solution. The silver nitrate will react with phosphate to make silver phosphate. After that, the additional quantity of silver nitrate is titrated with potassium thiocyanate. Thus, the total quantity of the silver nitrate is equivalent to the quantity of thiocyanate and phosphate ion used for the process.
Image courtesy: chemwiki.ucdavis.edu