Difference Between Tonicity and Osmolarity
Osmolarity and tonicity are the two terms that a person is bound to frequently encounter when dealing with different kinds of solutions.
The two terms are of utmost importance, especially in the medical field, where the tonicity and osmolarity plays a key role in keeping a person alive and healthy.
For those who are already familiar with tonicity and osmolarity, they would know that both the words refer to the relative concentration of solutes in two solutions.
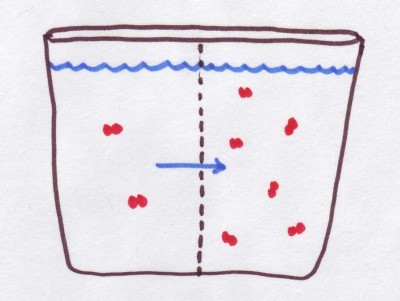
The two concepts normally pertain to the concentration of solutes inside a cell and outside of it, which in turn determines whether water would flow out of the cell into the surrounding solution, or move in to the cell from the outside solution.
Since tonicity and osmolarity are similar concepts, a lot of people find it pretty hard to differentiate between the two. However, there are still some subtle differences, which can make it possible to tell one from another.
One of the key differences between tonicity and osmolarity is that the latter refers to a state that is created when the total concentration of only non-penetrating solutes is taken into consideration i.e. the solutes that cannot move across the semi-permeable membrane separating the two solutions, whereas osmolarity takes both penetrating, as well as non-penetrating solutes into account.
Tonicity and osmolarity also differ from each other on account of how effective they are. The former can be considered as the osmotic pressure, while the latter is more inclined towards being the measure of the concentration of a solution.
Simply put osmolarity can help determine which, if any, solutes would move from one solution to another across the semi-permeable membrane separating them, whereas tonicity merely helps to determine whether water would flow into a given solution from its surrounding solution, or if it would lose its water to the outside solution.
Instructions
-
1
Tonicity
Tonicity is the measure of difference in concentrations of two solutions, taking only the non-penetrating solutes into account. It helps to determine the osmotic pressure gradient and therefore the direction in which the water would flow. If a cell is placed in a hypertonic solution, i.e. a solution with more non-penetrating solutes than the cell, water will flow from the cell into the solution, thus causing the cell to shrink and eventually shrivel. If it is placed inside a hypertonic solution, with low concentration of non-penetrating solutes, water will flow from the solution into the cell, causing it to increase in size and eventually burst.
Image courtesy: chemistry.about.com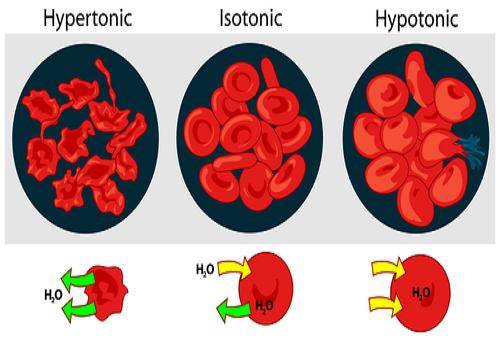
-
2
Osmolarity
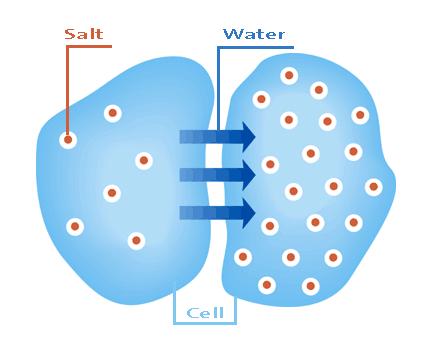
Osmolarity, also known as osmotic concentration, is the measure of the solute concentration of a solution. It can also be regarded as the osmotic pressure of a solution.
Image courtesy: mathbench.umd.edu