Difference Between Uranium and Plutonium
Uranium and plutonium are two naturally occurring radioactive elements, with the symbols U and Pu respectively. In an oxygen-free environment, uranium occurs as a silvery, metallic-gray coloured solid; however, it oxidizes in air to develop a dark-coloured, chalky coating of uranium oxide. Plutonium, on the other hand, naturally occurs as a silver-white solid; however, when exposed to air, it also reacts with oxygen, and its normal appearance gains a slight yellow tint.
Both plutonium and uranium are heavier than lead, with the former possessing 74 percent more mass per unit volume as compared to lead, and the latter being 68 percent denser than lead. Uranium, in a fine powder form, is pyrophoric, which means that in the presence of excess oxygen, it will burn spontaneously.
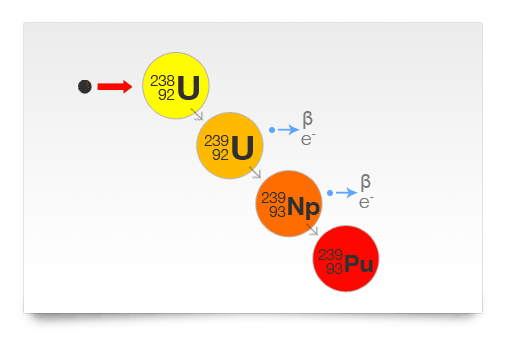
Uranium occurs naturally in the earth’s crust and has three naturally occurring isotopes – U-238, U-235 and U-234 – with U-238 being the most common isotope, accounting for more than 99 percent of the total deposits of uranium, while U-235 makes up 0.72 percent. Plutonium, on the other hand, rarely exists naturally, and is usually synthesised by exposing U-238 to neutron radiations. The entire process is carried out in a nuclear reactor, and the rays that are emitted during the process are extremely hazardous.
Since uranium and plutonium are radioactive elements, they both emit rays. Both these metals radiate similar kinds of radiations, called alpha particles, with very poor penetrating power. Uranium has only three electrons in the f sub shell, whereas plutonium has six.
Uranium isotopes have a much higher life time as compared to plutonium isotopes. U-235 has a half-life of about 700 million years, whereas plutonium-239 has a half-life of 24,000. One gram of Pu-239 gives off almost 30,000 times as much radiation as an equal amount of U-235 would give off.
Instructions
-
1
Uranium
Uranium is a silvery white radioactive metal placed in the actinide series of the periodic table, with the symbol “U” and the atomic number 92 (i.e., it has 92 electrons and 92 protons, and is the 92nd element of the periodic table). Uranium has six valence electrons, distributed in s, d and f sub shells or orbitals. Since uranium is a metallic element, it is hard, malleable, and ductile, but unlike other metals, it is a bad conductor.
Image courtesy: world-nuclear.org
-
2
Plutonium
Plutonium is a silvery-gray, transuranic, metallic, radioactive element, placed in the actinide series, with the symbol Pu and the atomic number 94. It is hard and brittle, and despite being a metal, it is a bad conductor of heat and electricity.
Image courtesy: auburn.edu