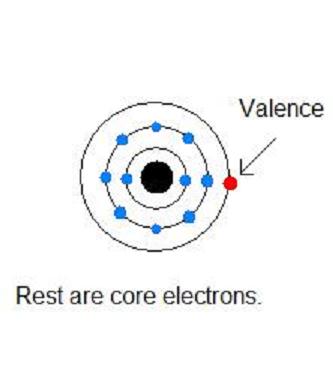
Difference Between Valence and Core Electrons
Learning about the structure of an atom can be useful and interesting as well, especially if you are a chemistry student. Remember that you will never pass a chemistry high school exam until you have a detailed knowledge about the atoms and their structures. Most people know very well that an atom consists of three particles, neutrons, protons and electrons. The center of an atom is called the nucleus which contains protons and neutrons, whilst the negatively charged electrons revolve around the nucleus in circular orbits. There can be a number of electrons shells or orbits around a nucleus and electrons within them have different attributes. It is extremely important to know the differences between valence electrons and core electrons.
Valence electrons are those present in the outermost shell of the atom (called the valence shell). Only valence electrons take part in any chemical reaction and form new products. These electrons are responsible of making bonds (ionic bonds and covalent bonds) with other reactants and resultantly, give an entirely different chemical.
Core electrons are all those electrons present in the inner shells of an atom, besides the valence shell. These electrons are relatively closer to the positively charged nucleus and hence, are not able to take part in the bonding process with other atoms. Core electrons might take part in chemical reactions only if all the valence electrons have already left their shell, making the core shell as the valence shell of the ion.
Instructions
-
1
Valence Electrons
Being farthest away from the attractive forces of the nucleus, valence electrons have the tendency to break away from their atom to form ions (in case of metals only). The number of valence electrons of an atom can be determined by looking at the Periodic Table of Elements. The Group number of the Periodic Table will denote the number of valence electrons in that particular atom, whereas the Period number will tell the number of shells that element has. While making an ionic bond, the valence electrons are lost to non-metals and the outermost shell is eliminated. The next core shell then becomes the valence shell but the atom will convert into an ion with a positive charge on it.
Image Courtesy: webanswers.com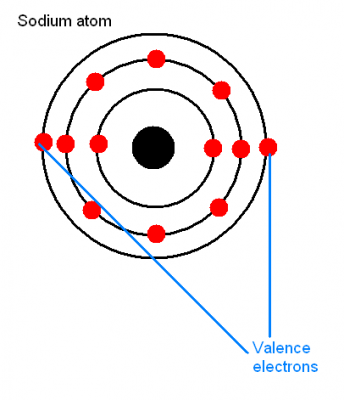
-
2
Core Electrons
These electrons do not take part in the chemical reactions and tend to screen the valence electrons from the attractive force of nucleus. Once the valence electrons are donated in a chemical reaction, the positive attraction of nucleus increases significantly and it make extremely hard for the core electrons to break away from their shells.
Image Courtesy: chemwiki.ucdavis.edu