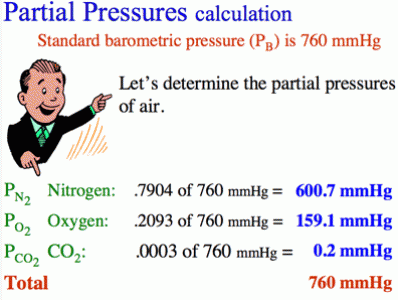
Difference Between Vapor and Partial Pressure
Vapor pressure and partial pressure are two important terms used when describing the properties of a gaseous system. Though both these terms describe the pressure exerted by gas that is enclosed in a container, they are two distinct terminologies. Vapor pressure is the property of a liquid that has vaporized in a closed system, whereas partial pressure is the property of a mixture of gases. Vapor pressure is the pressure exerted by the vapors of a substance that are in equilibrium with the condensed phase, be it a solid or a liquid, in a closed system, at a given temperature and pressure. Partial pressure, on the other hand, is the pressure exerted by a single gas in a mixture of gases.
Both vapor pressure and partial pressure require closed systems for their measurement. Vapor pressure is measured in Pascal, whereas partial pressure is measured in “kPa” or “mmHg”.
Instructions
-
1
Vapor pressure
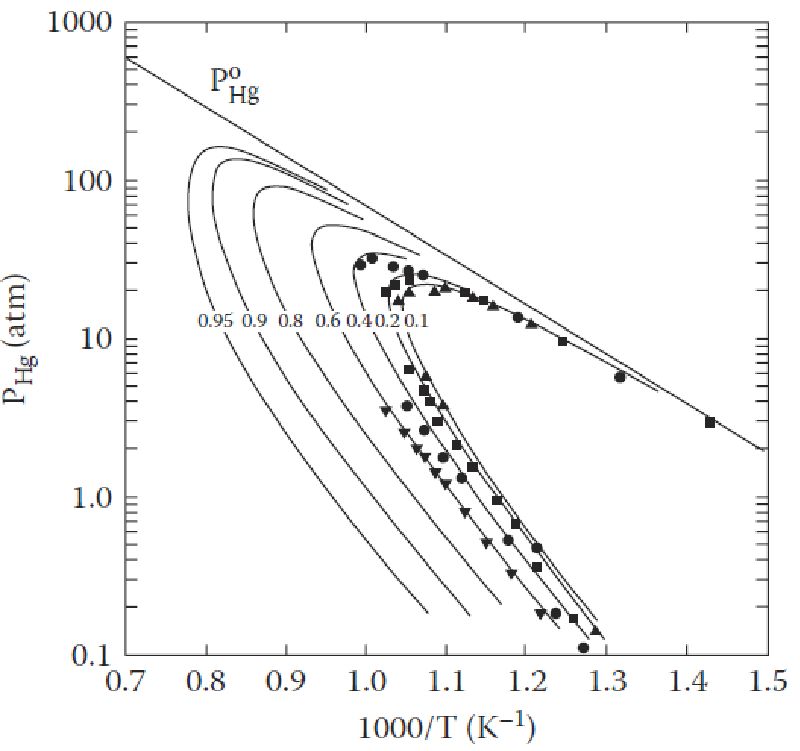
Vapor pressure is defined as “the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system”. The equilibrium vapor pressure indicates the evaporation rate of a liquid, since it is related to the tendency of particles to escape from the surface of a liquid or solid (when particles are escaping from the surface of a solid, the process is called sublimation). A solid or a liquid that has high vapor pressure under normal conditions is said to be volatile.
There are two types of vapor pressures; namely, saturated vapor pressure and unsaturated vapor pressure. Saturated vapor pressure is the pressure exerted by vapors in a closed system that contains both the vapors and the condensed phase of the substance, usually liquid. An unsaturated system, meanwhile, is one that consists of vapors only; any liquid or solid added to this will evaporate, until the saturation point is reached. The pressure exerted by the vapors in an unsaturated system is known as unsaturated vapor pressure.
Temperature does affect the vapor pressure of a substance and bears a positive relationship, but it increases non-linearly with temperature, i.e. the two do not bear a one-to-one relationship.
Image courtesy: kidsgeo.com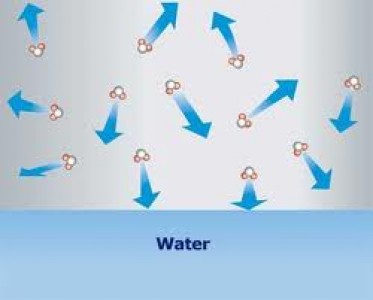
-
2
Partial Pressure
The partial pressure of a system containing a mixture of ideal gases is defined as “the hypothetical pressure of that gas if it alone occupied the volume of the mixture at the same temperature”. Partial pressure is a fractional, dimensionless value that can only vary in the range of 0 to 1. The total pressure of the gas mixture can be obtained by adding the partial pressures of all the gases in the mixture.
Image courtesy: unm.edu