How to Make Electrochemical Cell
Everyone knows how a battery cell works. Apart from cell driven toys, there are various home appliances in which these cells are used. Basically, the working of the cell involves certain chemicals that have the tendency to conduct electricity. Hence, a simple Electrochemical cell can be made with the help of some basic tools. This can serve as a good idea, infact a handy substitute, for primary school students who are looking for Science project ideas. The good thing about this experiment is that it is simple to perform and involves minimum expenses. However, you need to have the below given available;
Required Items:
– Two glass beakers or containers
– Copper Sulphate
– Zinc Sulphate
– Table Salt
– Distilled Water
– Zinc and Copper Strips
– U shaped plastic tube
– Cotton pieces
– Voltmeter
Instructions
-
1
Getting Started
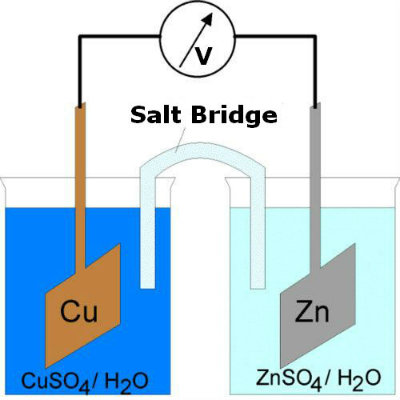
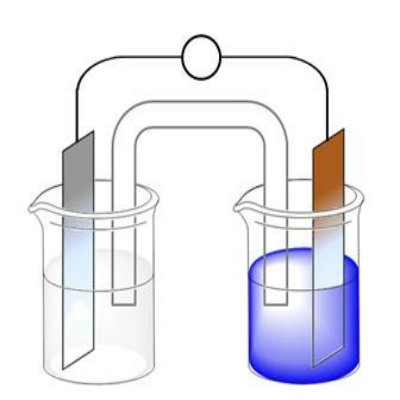
Place two beakers at some distance from each other, adding Zinc Sulphate in one and Copper Sulphate in the other.
-
2
Making the solutions
Now add distilled water to both beakers in a ratio that you have 0.35 to 1.05 ounces of chemical in every seven tablespoons of water. -
3
The Zinc and Copper Strips
After you are done with making the solutions, insert Zinc strip into the Zinc Sulphate solution and the Copper strip in Copper Sulphate solution.

-
4
The Salt Bridge
This is a bridge that you prepare by preparing a salt solution with 0.35 ounces of table salt dissolved in seven tablespoons of water. The solution is put inside the U shaped plastic tube which is provided with cotton wads stoppers on both ends. Finally, both ends of the tube should be immersed in each of the beakers in form of a bridge. -
5
Connecting the Voltmeter
In order to ensure that the Electrochemical cell is complete, connect a voltmeter between the Zinc and Copper strips. If the current is flowing, there will be deflection or reading on the voltmeter. The voltage produced may not be very high but it can be increased by adding more such cells.