How to Write Electron Configurations for Molecules
The atom of any chemical element consists of nucleus and electrons. The number of electrons in an atom depends on its atomic number. Thus, the electronic configuration of molecules determines the distribution of electrons in the shells and sub shells.
Molecules are the smallest particles of a chemical substance. They have a constant number of atoms, united by chemical bonds. The chemical identity of the molecule is expressed by a set of configuration and the chemical bonds between its atoms.
Molecules are made of atoms. Their location can be transmitted by means of structural formulas. Some molecules, such as proteins, can contain hundreds of thousands of atoms.
You need to know the octet rule before writing electronic configuration. The rules states that an atom participating in the formation of chemical bonds (giving or taking electrons) tries to acquire the electron configuration of an inert gas – octet (eight) electrons.
Electronic formula molecules (ions and free radicals) are widely used in organic chemistry. However, they do not reflect the spatial structure of molecules.
Eventually chemists have realized that there is not a single chemical formula for precise characteristics of the molecule, since there are molecule isomers having the same chemical formula but different properties.
Instructions
-
1
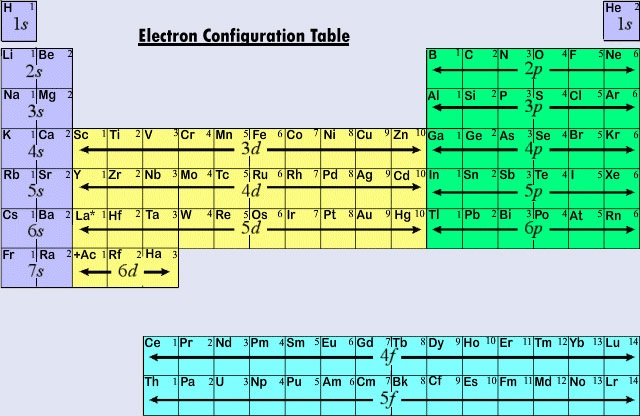
First, you have to find the atomic number of the atom and this number should be equal to the number of electrons. The electrons in an atom occupy the available orbitals in sequence, called the scale of energy: 1s / 2s, 2p / 3s, 3p / 4s, 3d, 4p / 5s, 4d, 5p / 6s, 4d, 5d, 6p / 7s, 5f, 6d, 7p.
-
2
The structure of the electron shells can be determined with the help of electronic graphic formulas. To write the formula using a matrix, you have to place one or two electrons with opposite spins in each shell. Electrons are represented by arrows. The matrix clearly shows that the s-orbital of the two electrons can be placed on the p-orbitals.
-
3
In general, the filling of the energy levels of electrons occurs in the following sequence: 1s2, 2s2, 2p6, 3s2, 3p6, 4s1 and so on. Thus for s-shell – not more than two electrons (one orbital); for p-shell - not more than six electrons (three orbital); for d-sublayer - not more than 10 (five orbitals); and for f-sublayer - not more than 14 (seven orbitals).
-
4
Write down the serial number and the symbol of the element next to the matrix. In accordance with the scale of the energy fill in series 1s, 2s, 2p, 3s, 3p, 4s levels inscribing two electrons in the cell. Get 2 +2 +6 +2 +6 +2 = 20 electrons.




